MSLRDT012 Tuberculosis Rapid Test Cassette
A rapid test for the qualitative detection of anti-TB antibodies (Isotypes IgG, IgM and IgA) in whole blood, serum or plasma specimens.
For professional in vitro diagnostic use only.
【INTENDED USE】
The Tuberculosis Rapid Test Cassette (Whole Blood/Serum/Plasma) is a rapid chromatographic
immunoassay for the qualitative detection of anti-TB antibodies (Isotypes IgG, IgM and IgA) in whole
blood, serum or plasma specimens.
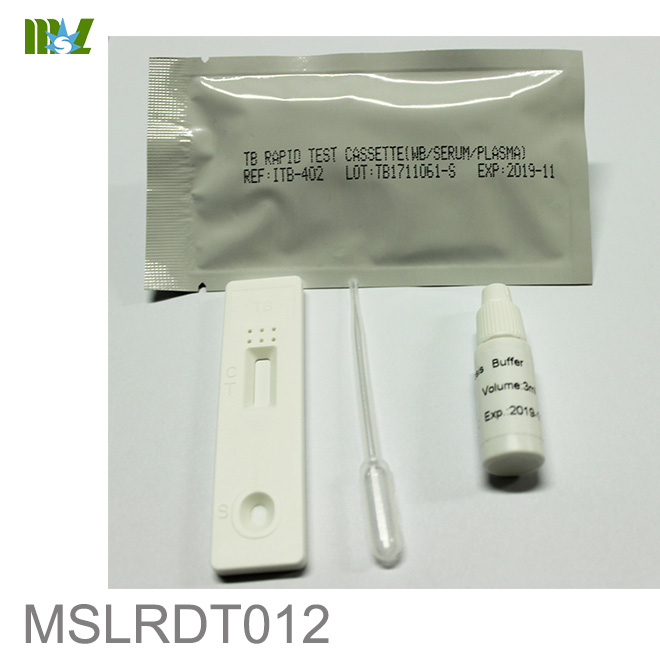
MSLRDT012 Tuberculosis Rapid Test Cassette
1. Fast.
2. High sensitivity and specificity.
3. Simple to use.
4. Accurate and reliable.
5. Ambient storage.
6. IgG, IgM and IgA can be detected.
|
Catalog No.
|
MSLRDT012
|
|
Product name
|
Tuberculosis Rapid Test Cassette (Whole Blood/Serum/Plasma)
|
|
Analyte
|
Isotypes IgG, IgM and IgA
|
|
Test method
|
Colloidal Gold
|
|
Sample type
|
WB/Serum/Plasma
|
|
Sample volume
|
3 drops
|
|
Reading time
|
10 mins
|
|
Sensitivity
|
86.40%
|
|
Specificity
|
99.0%
|
|
Storage
|
2~30℃
|
|
Shelf life
|
24 months
|
|
Qualification
|
CE
|
|
Format
|
Cassette
|
|
Package
|
40T/kit
|
MSLRDT012 Tuberculosis Rapid Test Cassette
【PRINCIPLE】
The Tuberculosis Rapid Test Cassette (Whole Blood/Serum/Plasma) is a qualitative, solid phase,
two-site sandwich immunoassay for the detection of anti-TB antibodies in whole blood, serum or
plasma specimens. The membrane is pre-coated with TB recombinant antigen on the test line region
of the Cassette. During testing, the anti-TB antibodies, if present in whole blood, serum or plasma
specimen react with the particles coated with TB recombinant antigen. The mixture migrates upward
on the membrane chromatographically by capillary action to react with TB recombinant antigen on the
membrane and generate a colored line. The presence of this colored line in the test region indicates a
positive result, while its absence indicates a negative result. To serve as a procedural control, a
colored line will always appear in the control line region indicating that proper volume of specimen
has been added and membrane wicking has occurred.
【PRECAUTIONS】
For professional in vitro diagnostic use only. Do not use after expiration date.
Do not eat, drink or smoke in the area where the specimens or kits are handled.
Do not use test if the package is damaged.
Handle all specimens as if they contain infectious agents. Observe established precautions
against microbiological hazards throughout testing and follow the standard procedures for proper disposal of specimens.
Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are being tested.
Humidity and temperature can adversely affect results.
The used test should be discarded according to local regulations.
Do not use potassium oxalate as anticoagulant to collect plasma or venous blood samples
MSL TEAM picture


MSL Certificate
MSL Medical cooperate with DHL,FEDEX,UPS,EMS,TNT,etc.International shipping company,make your goods arrive destination safely and quickly.





 Price is 8-20% Lower Than Other
Price is 8-20% Lower Than Other














![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180522/201805221842185093.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041722259316.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801051008471506.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041743356476.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801050937087001.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801050937098646.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/20180104151925823.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041722253617.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180522/201805221832489592.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041743355792.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801051008472478.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041722257608.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801050937095861.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041743356059.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041519256624.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041519253116.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180111/201801111705377686.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801051008478805.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180614/201806141752243474.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20200412/202004122124098335.jpg.jpg)


